Computer Chemistry Consultancy - XML4Pharma, Katzelbachweg 18, A-8052 Thal, Austria, info@CompChemCons.com
Hoy Solubility Parameter Calculation
Other related software: calculation of solubility parameters of mixtures
The method is essentially a group contribution method: for each chemical group in the molecule, contributions are added to the total. The Hoy Solubility Parameter method is special as it allows to "correct" for structural features like: cis, trans (around double bonds), ortho-, meta-, para-substitution (aromatics), branching (isopropyl, t-butyl, conjugation of double bonds, and rings.
who is using this software ...
|
|
|
|
|
The Hoy Solubility Parameter application
Our application allows the calculation of each of the three components (and the total) of the solubility parameter, which can then be used to predict whether the material is compatible with another material.
It includes a powerfull feature to search in a database of over 750 solvents and polymers, in order to find the most suitable solvent(s) and compatible polymers.
The graphical user interface is very intuive and easy to use. Just click on the buttons of the groups that are present in your molecule or polymer, and enter the number of these groups. If you know the denisty of your material, you can enter it. If not, the software calculates it. |
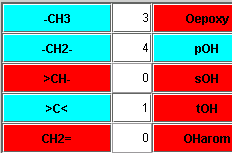 |
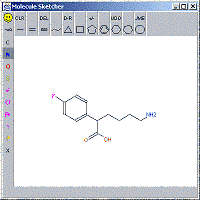 |
Alternatively, you can use the molecule sketcher, the program then breaks down the molecule into Hoy groups (report is given) after which you still can make adjustments in the main panel. |
 |
After having performed a classic calculation, and clicking the "Search best solvents" button, the database is opened and the materials in the databased are sorted. The sorting is based on the distance ("difference") in 3-dimensional space (with the axes being the "polar", the "hydrogen bonding" and the "dispersive" contributions to the solubility parameter (see theory). The N best solvents (according to your choice in the input field) are then displayed in a table which also lists other properties of the solvents. By clicking on a column label you can sort the table according to the column property (one click from small to large, the next click from large to small). |
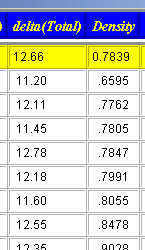 |
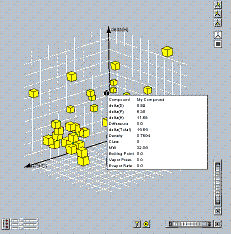 |
These solvents are also plotted in a 3-dimensional plot (rotatable and zoomable), and filtered according to minimum and maximum values of any of the other properties that are in the database (e.g. vapor pressure, boiling point) etc.. |
Features and Screenshots
All the features and many screenshots ...
Flexibility
Our web application is highly flexible:
- It comes with an XML-file which contains all the Hoy group parameter data. This means that you can add, delete or change (individual parameters of) molecular groups (at your own risk). This is very powerful as it allows you to use your own (propriety) set of parameters and groups.
- You can add, delete, or change data in the database with solvents and materials. If you have a propriety company database with materials, we can provide you with a suitable interface.
- You can change colors of the components (e.g. the background color) to your own taste.
- You can change the size of the labels in the buttons with the groups.
- You can print out the results of the calculation and of the search for the best solvents
Technical implementation
This web application comes either as a Java applet or a Java standalone application.
Web Implementation: The software comes as a Java applet.
This means that you implement the software on your own (department, insitute, company) web server.
If this webserver is generally accessible for all scientists in your company, this
means that they all can use this application !
Compare this with much more expensive applications that come as single-PC
licenses ! Our licensing policy allows you to implement the application
on one web server on your intranet, even if this web server is accesible by
thousands of coworkers in your company.
As this application comes as an applet, it is very easily installed: just copy all the files from the distribution to a directory under the webroot directory of your webserver (e.g. htdocs for an Apache web server)
Standalone Implementation: The software can also be obtained as a standalone Java application. Also here, installation is very easy. The functionality of the standalone application is identical to that of the web application.
Some of the users of this software
- The central R&D labs of a major Coatings and Resins company uses this software to predict the compatibility between their resins and organic solvents. Before they had access to this technology, many of their newly developed resins did not meet the solubility requirements. The software now helps them to pre-select newly envisaged resins, even BEFORE actually synthesizing them, thus saving them several man-years of R&D.
- A major chemical company selling organic additives for PVC (stabilizers, antioxydants) uses the software to predict the compatibility of new envisaged organic molecules with PVC. Before they had this software, they needed a lot of experimental work (synthesis, long-term testing in PVC strips), to find out whether a new additive would not phase-separate in PVC. With the software, they easily can predict this, thus making research much more efficient.
- A Scandinavian company active in the oil-drilling industry, uses an adapted version of our sofware to predict whether certain polymers used in the oil-drilling industry do or do not swell when in contact with an oil mixture. This allows them to select the right polymer for the right application, with much less testing than ever before.
- The software is used by a company that develops new materials for use in the dental industry. Before they had this software, they had no idea how to predict whether a new envisaged material would be compatible with other materials, or which solvent to choose to solubilize the material.
- A company that markets environment-friendly solvents deployed the software on the notebooks of its technical sales people. As such, when the salesperson visit a potential customer, it can immediately be calculated whether the material of the potential customer will be soluble or compatble with one of the environment-friendly solvents that the company markets.
References:
- K.L.Hoy, New values of the solubility parameters from vapor pressure data, J.Paint Techn., Vol.42, Nr.541, p.76 (1970).
- K.L.Hoy, The Hoy tables of solubilty parameters, Union Carbide Corp., 1985
- K.L.Hoy, Solubility Parameters as a design parameter for water borne polymers and coatings. Preprints 14th Int.Conf. Athene, 1988.
- K.L.Hoy, J.Coated Fabrics, 19, p.53 (1989)
Scientific publications:
The following is a list of scientific publications about research in which the authors used our software:
- Effect of resin hydrophilicity and water storage on resin strength
C.K.Y. Yiua, N.M. Kinga, D.H. Pashley, B.I. Suh, R.M. Carvalho, M.R.O. Carrilho and F.R. Tay,
Biomaterials, Volume 25, Issue 26, November 2004, Pages 5789-5796. - Correlation of Bond Strengths of Self-etching Cement Systems with Solubility-Parameters,
U. Salz (Ivoclar Vivadent AG, Switzerland),
The IADR/AADR/CADR 83rd General Session, Baltimore, 2005 - Effects of Resin Hydrophilicity on Dentin Bond Strength
Y. Nishitani1, M. Yoshiyama1, A.M. Donnelly, K.A. Agee, J. Sword, F.R. Tay, D.H. Pashley,
Journal of Dental Research, November 2006, vol. 85 no. 11, Pages 1016-1021. - Use of Hoy's solubility parameters to predict water sorption/solubility of experimental primers and adhesives.
Yoshihiro Nishitani1, Masahiro Yoshiyama1, Keiichi Hosaka, Junji Tagami, Adam Donnelly, Marcela Carrilho, Franklin R. Tay, David H. Pashley,
European Journal of Oral Sciences, Volume 115, Issue 1, pages 81–86, February 2007. - From dry bonding to water-wet bonding to ethanol-wet bonding. A review of the interactions between dentin matrix and solvated resins
using a macromodel of the hybrid layer
DAVID H. PASHLEY, DMD, FADM, PHD, FRANKLIN R. TAY, BDSC, (HONS), FADM, PHD, RICARDO M. CARVALHO, DDS, PHD, FREDERICK A. RUEGGEBERG, DDS, MS, KELLI A. AGEE, BS, MARCELA CARRILHO, DDS, PHD, ADAM DONNELLY, BS & FRANKLIN GARCÍA-GODOY, DDS, MS
Am. J. Dent., 2007, Vol. 20, Pages 7-21 - Durability of Resin-Dentin Bonds to Water- vs. Ethanol-saturated Dentin,
K. Hosaka, Y. Nishitani, J. Tagami, M. Yoshiyama, W.W. Brackett, K.A. Agee, F.R. Tay and D.H. Pashley
J. Dent. Res. 2009, Vol. 88, Page 146