Computer Chemistry Consultancy - XML4Pharma, Katzelbachweg 18, A-8052 Thal, Austria, info@CompChemCons.com
Ricin, a toxic protein
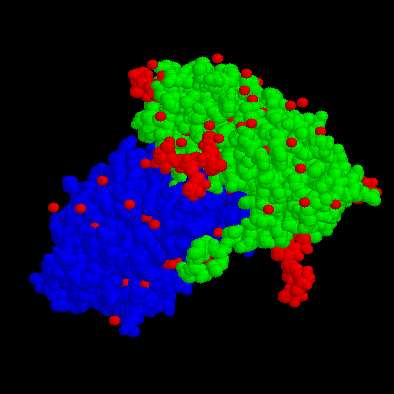
The toxin Ricin has been in the news very recently, due to the messages that terroristic groups are preparing large amounts of this very toxic material (one milligram can kill an adult), to use it in chemical warfare. What the news messages do not tell us however, is that Ricin is a natural product, from the oil of the castor bean. Ricin is a so-called "ribosome-inactivating protein", inhibiting protein synthesis in the cell. The molecular structure of this protein is given below, once as a CPK image, and one showing the folds and chains of the protein.
 |
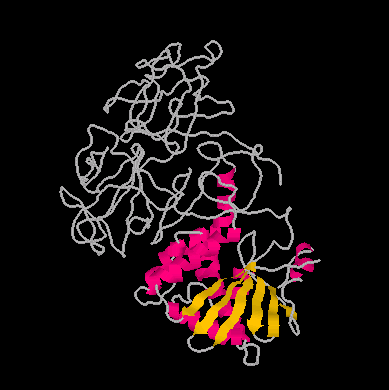 |
The Ricin molecular structure consits of two disulfide linked subunits (each about 30,000 MW), called the A- and the B-chain. The A-chain is composed of 267 amino acid residues and is classed as an N-glycosidase. The toxically active A chain of ricin is approximately 30% helical and contains 7 alpha helices. It also contains about 15% beta structure which is made up of a five stranded beta sheet. The B-chain is composed of 262 amino acid residues and is classed as a lectin. The B chain has an affinity for bindig to galactosides and posseses two galactose binding sites that are attracted to galactose containing glycoprotiens at the cell surface. The A and B chain glycoproteins are linked by a disulfide bond located at residue 259 of the A chain and residue 4 of the B chain.
Ricin can easily be extracted from the Castor bean by cold or hot pressing.
Those expecting high-tech labs for the "synthesis" of Ricin, must thus be disappointed.
It would be more appropriate to call Ricin "the poor terrorist's chemical weapon".